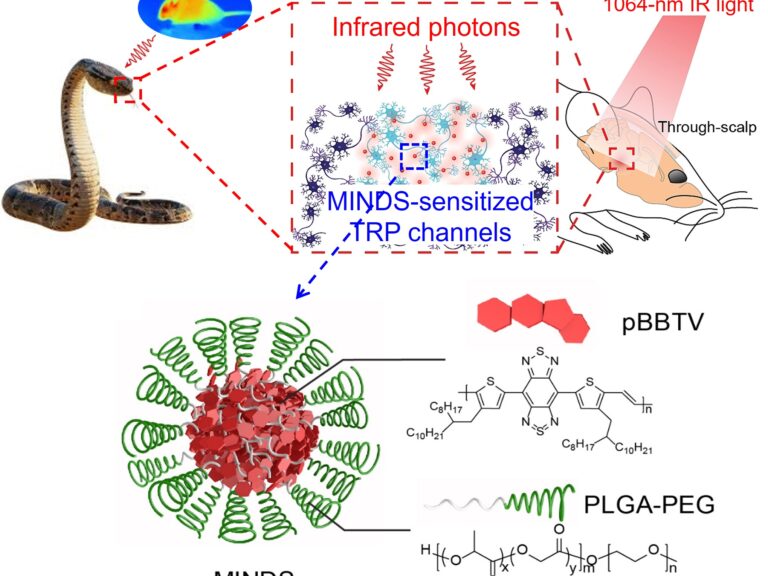
Optogenetics enables causal dissection of neural circuitry underlying particular behaviors and disorders with neuron-type specificity. The implementation of optogenetics in live mice usually requires an optical fiber to deliver visible light in the brain with two critical challenges. First, the implanted fiber causes acute damage and chronic immune responses in the brain tissue. Second, the fiber tethers the animal and results in steric constraints interfering with animals’ natural movements. To mitigate these challenges, we aim to develop optogenetics in the second near-infrared (NIR-II) spectrum (1,000-1,700 nm), which has been demonstrated to offer deeper tissue penetration than its shorter-wavelength counterparts. We leverage two Nobel-winning findings in our research. First, we learn from the molecular basis of infrared detection by snakes to impart NIR-II sensitivity to specific neurons in the mouse brain. Second, we use conjugated semiconducting polymers with precise bandgap control to further sensitize these neurons to low-intensity NIR-II light. With this approach, we envisage an implant-free and tether-free optical interface for modulating neural activity in freely moving mice engaged in individual and social behaviors. Read our papers in Nature Biomedical Engineering ACS Nano STAR Protocols to learn more.