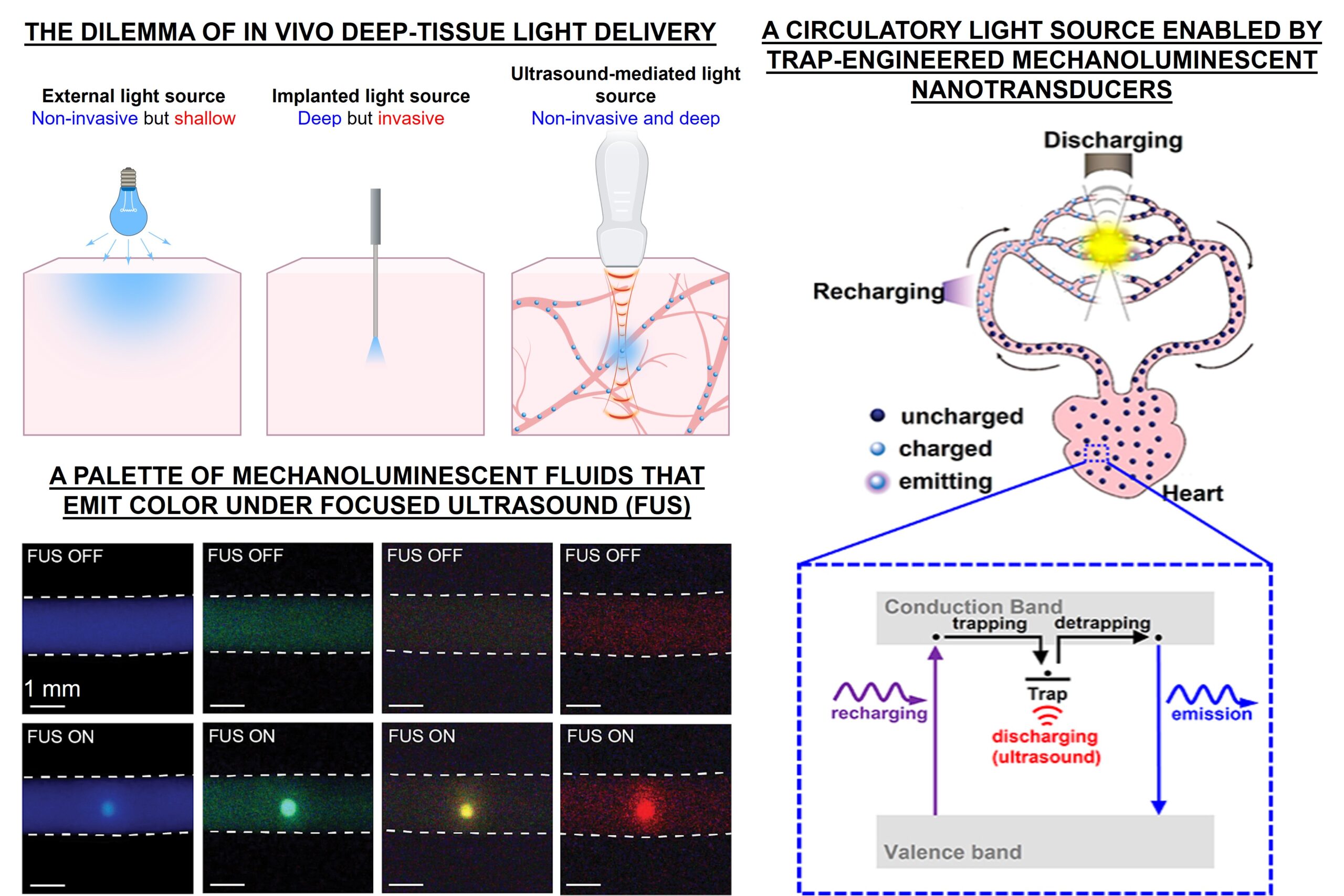
Light is used in a wide range of methods in chemistry, biology, and medicine, such as photosynthesis, fluorescence imaging, optogenetics, and light-activated therapy for cancers and viral infections. A critical challenge of applying light in vivo, such as deep-brain optogenetic neuromodulation and photochemotherapy in deep organs, is the poor penetration of photons in biological tissue due to scattering and absorption. Therefore, delivering light deep into the body requires invasive procedures, such as the insertion of optical fibers, as well as surgical dissection of overlying tissues. The very invasiveness of these procedures also precludes easy repositioning of the illuminated region in the same subject. Leveraging the endogenous circulatory system in the body and systemically delivered phosphors, we aim to develop a general tool of noninvasive light delivery for any biological applications that need light deep inside the body. Specifically, mechanoluminescent nanotransducers are delivered systemically into the circulatory system. They act as “optical flow batteries”, transporting recharging light that is incident on the skin to local light emission in deep tissues (e.g., brain, liver, and lungs). This local light emission is gated by tissue-penetrant ultrasound, which can be focused and scanned in the biological tissue in vivo at any location or depth whenever needed. Read our papers in PNAS Science Science Advances JACS JACS Nanoscale Advanced Drug Delivery Reviews to learn more. A patent has been filed and published on this technology.